

Substantial Reductions in Fasting Triglycerides, HbA1c, and Fasting Glucose
as Early as 4 Months & Sustained Over 12 Months4
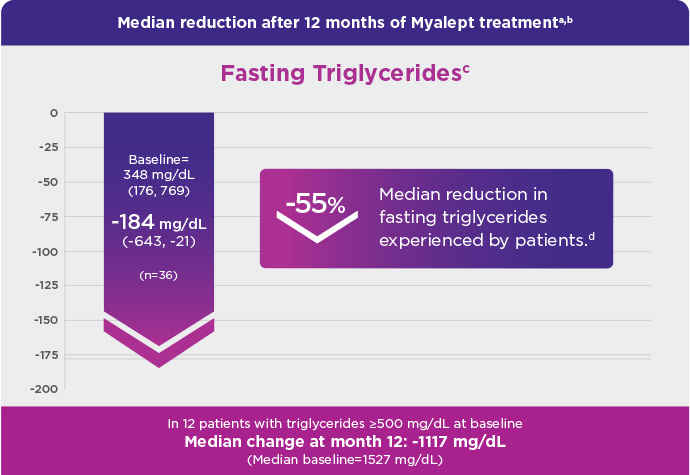
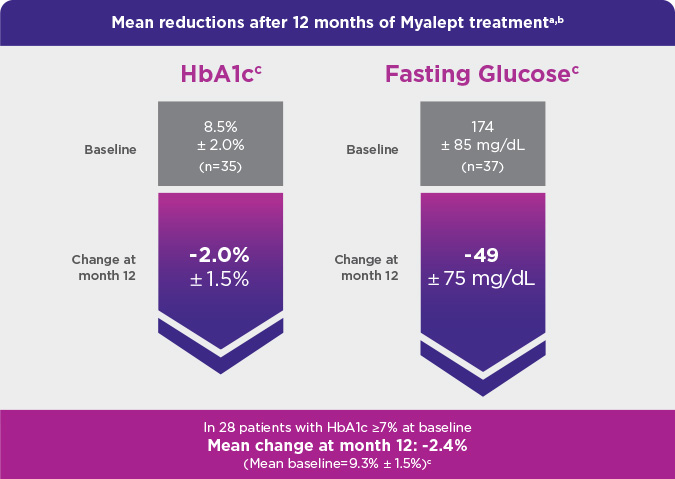
- This phase 3, open-label, single-arm study evaluated Myalept treatment in 48 patients. Patients had to have congenital generalized lipodystrophy (CGL) or AGL and diabetes mellitus, hypertriglyceridemia, and/or increased fasting insulin.
- Of the 48 patients enrolled, 32 (67%) had CGL, 16 (33%) had AGL, 36 (75%) were female, 22 (46%) were Caucasian, 10 (21%) were Hispanic, and 9 (19%) were Black.
- The median age at baseline was 15 years (range: 1-68 years), with 35 (73%) patients less than 18 years of age.
- The median fasting leptin concentration at baseline was 0.7 ng/mL in males (range: 0.3-3.3 ng/mL) and 1.0 ng/mL in females (range: 0.3-3.3 ng/mL).
- The median duration of Myalept treatment was 2.7 years (range: 3.6 months-10.9 years).
- The n numbers across the 3 metabolic parameters—fasting triglycerides (n=36), HbA1c (n=35/36), and fasting glucose (n=37)—differ from the total N number (N=48) because not all patients were able to contribute data to each parameter (fasting triglycerides, HbA1c, and fasting glucose) and/or each time point (baseline and month 12).
- Fasting triglycerides: mg/dL (quartile 1, quartile 3); HbA1c: % ± standard deviation (SD); fasting glucose: mg/dL ± SD.
- Quartiles 1 and 3 of median percent change were -77% and -20%, respectively.
Additional Data Supporting Key Myalept Efficacy5
Safety and effectiveness of Myalept were also evaluated in a final combined analysis of the phase 3 study.5 These data shown represent secondary endpoints of the trial.
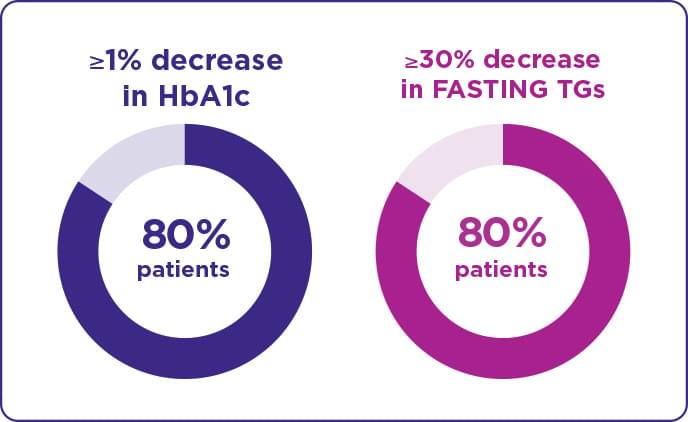
At month ≥12, 80% of patients had a ≥1% decrease in HbA1c or ≥30% decrease in TGs.
- Significant outcomes were seen as early as Month 4
Additionally, more than one-third of patients achieved the highest target reductions of both a ≥2% reduction in HbA1c AND ≥40% reduction in TGs.
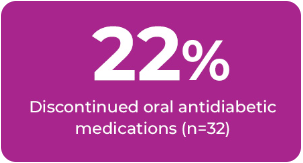
Of those patients on medications:
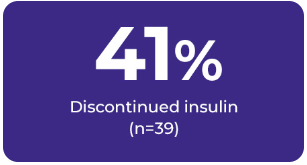

In addition: In a subset of GL patients with liver imaging data:
Mean decrease in liver volume at month 12 was 33.8% (p <0.001, n =12)
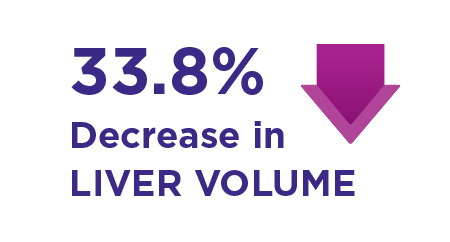
The safety and effectiveness of Myalept for the treatment of liver disease, including NASH, have not been established.
A retrospective study evaluated both clinical and humanistic consequences of GL and treatment. Chart data were abstracted from a cohort of Myalept-treated patients with GL (n=68) treated at the US National Institutes of Health (NIH)6
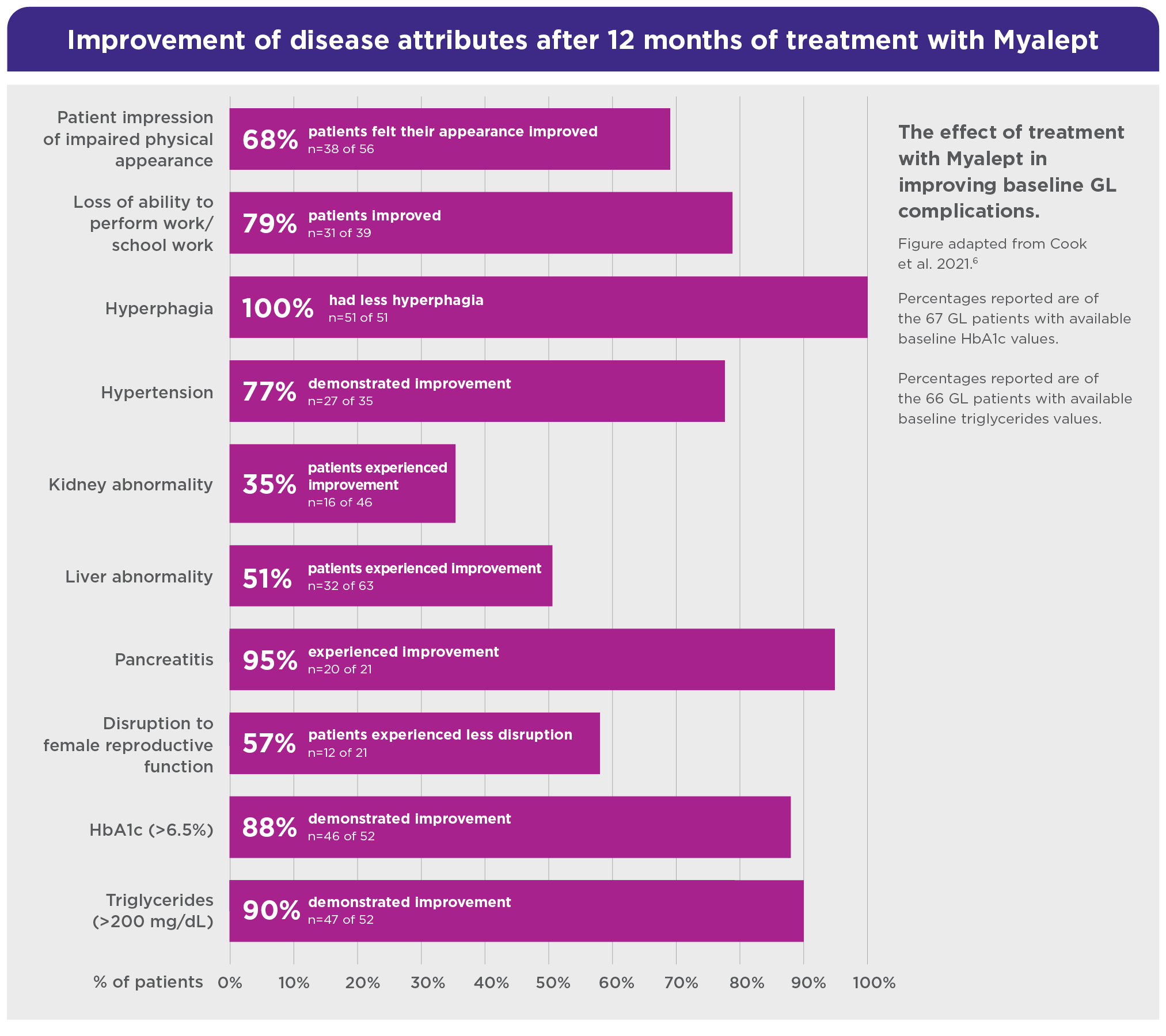
In this post hoc analysis study, Myalept was associated with meaningful clinical and quality of life improvements.6
4. Myalept® (metreleptin) for injection for subcutaneous use [package insert]. Chiesi Pharmaceuticals DAC; 2021.
5. Brown RJ, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 2018; 60(3): 479–489.
6. Cook K, et al. Effects of Metreleptin on patient outcomes and quality of life in generalized and partial lipodystrophy. J Endocrin Soc 2021; 5(4): 1–16.
INDICATION: MYALEPT® (metreleptin) for injection is a leptin analog indicated as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy.
LIMITATIONS OF USE: The safety and effectiveness of MYALEPT for the treatment of complications of partial lipodystrophy or for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established.
MYALEPT is not indicated for use in patients with HIV-related lipodystrophy. MYALEPT is not indicated for use in patients with metabolic disease, including diabetes mellitus and hypertriglyceridemia, without concurrent evidence of generalized lipodystrophy.
WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA
Anti-metreleptin antibodies with neutralizing activity have been identified in patients treated with MYALEPT. The consequences of these neutralizing antibodies are not well characterized but could include inhibition of endogenous leptin action and/or loss of MYALEPT efficacy. Severe infection and/or worsening metabolic control have been reported. Test for anti-metreleptin antibodies with neutralizing activity in patients who develop severe infections or show signs suspicious for loss of MYALEPT efficacy during treatment. Contact Chiesi Farmaceutici S.p.A. at 1-866-216-1526 for neutralizing antibody testing of clinical samples.
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy, both treated and not treated with MYALEPT. Carefully consider the benefits and risks of treatment with MYALEPT in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy.
Because of these risks associated with the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or MYALEPT and the risk for lymphoma, MYALEPT is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the MYALEPT REMS PROGRAM.
CONTRAINDICATIONS: MYALEPT is contraindicated in general obesity not associated with congenital leptin deficiency. MYALEPT has not been shown to be effective in treating general obesity. The development of anti-metreleptin neutralizing antibodies have been reported in obese patients treated with MYALEPT. MYALEPT is contraindicated in patients with prior severe hypersensitivity reactions to metreleptin or to any of its components.
WARNINGS AND PRECAUTIONS: A dose adjustment, including possible large reductions, of insulin or insulin secretagogue may be necessary in some patients to minimize risk of hypoglycemia. Closely monitor blood glucose in patients on concomitant insulin, especially those on high doses, or insulin secretagogue.
Cases of progression of autoimmune hepatitis and membranoproliferative glomerulonephritis (associated with massive proteinuria and renal failure) were observed in some patients with acquired generalized lipodystrophy treated with MYALEPT. A causal relationship between MYALEPT and the development and/or progression of autoimmune disease has not been established. Carefully consider the benefits and risks of MYALEPT treatment in patients with autoimmune disease.
Hypersensitivity reactions (eg, anaphylaxis, urticaria or generalized rash) have been reported. Patient should promptly seek medical advice about discontinuation of MYALEPT if a hypersensitivity reaction occurs.
MYALEPT contains benzyl alcohol when reconstituted with Bacteriostatic Water for Injection. The preservative benzyl alcohol has been associated with serious adverse events and death in pediatric patients, particularly in neonates and premature infants. Preservative-free Water for Injection is recommended for use in neonates and infants.
ADVERSE REACTIONS: Most common adverse reactions (≥10%) in clinical trials were headache, hypoglycemia, decreased weight, and abdominal pain.
Please see full Prescribing Information, including Boxed Warning.
INDICATION: MYALEPT® (metreleptin) for injection is a leptin analog indicated as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy.
LIMITATIONS OF USE: The safety and effectiveness of MYALEPT for the treatment of complications of partial lipodystrophy or for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established.
MYALEPT is not indicated for use in patients with HIV-related lipodystrophy. MYALEPT is not indicated for use in patients with metabolic disease, including diabetes mellitus and hypertriglyceridemia, without concurrent evidence of generalized lipodystrophy.
WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA
Anti-metreleptin antibodies with neutralizing activity have been identified in patients treated with MYALEPT. The consequences of these neutralizing antibodies are not well characterized but could include inhibition of endogenous leptin action and/or loss of MYALEPT efficacy. Severe infection and/or worsening metabolic control have been reported. Test for anti-metreleptin antibodies with neutralizing activity in patients who develop severe infections or show signs suspicious for loss of MYALEPT efficacy during treatment. Contact Chiesi Farmaceutici S.p.A. at 1-866-216-1526 for neutralizing antibody testing of clinical samples.
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy, both treated and not treated with MYALEPT. Carefully consider the benefits and risks of treatment with MYALEPT in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy.
Because of these risks associated with the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or MYALEPT and the risk for lymphoma, MYALEPT is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the MYALEPT REMS PROGRAM.
CONTRAINDICATIONS: MYALEPT is contraindicated in general obesity not associated with congenital leptin deficiency. MYALEPT has not been shown to be effective in treating general obesity. The development of anti-metreleptin neutralizing antibodies have been reported in obese patients treated with MYALEPT. MYALEPT is contraindicated in patients with prior severe hypersensitivity reactions to metreleptin or to any of its components.
WARNINGS AND PRECAUTIONS: A dose adjustment, including possible large reductions, of insulin or insulin secretagogue may be necessary in some patients to minimize risk of hypoglycemia. Closely monitor blood glucose in patients on concomitant insulin, especially those on high doses, or insulin secretagogue.
Cases of progression of autoimmune hepatitis and membranoproliferative glomerulonephritis (associated with massive proteinuria and renal failure) were observed in some patients with acquired generalized lipodystrophy treated with MYALEPT. A causal relationship between MYALEPT and the development and/or progression of autoimmune disease has not been established. Carefully consider the benefits and risks of MYALEPT treatment in patients with autoimmune disease.
Hypersensitivity reactions (eg, anaphylaxis, urticaria or generalized rash) have been reported. Patient should promptly seek medical advice about discontinuation of MYALEPT if a hypersensitivity reaction occurs.
MYALEPT contains benzyl alcohol when reconstituted with Bacteriostatic Water for Injection. The preservative benzyl alcohol has been associated with serious adverse events and death in pediatric patients, particularly in neonates and premature infants. Preservative-free Water for Injection is recommended for use in neonates and infants.
ADVERSE REACTIONS: Most common adverse reactions (≥10%) in clinical trials were headache, hypoglycemia, decreased weight, and abdominal pain.


