Becky, Myalept patient,
Becky, Myalept patient,is a paid spokesperson for Chiesi
Myalept Dosing Information
Myalept Is a Once-Daily Subcutaneous Injection
- Myalept should be administered once daily at the same time every day, regardless of the timing of meals1
- If a dose is missed, patients should be instructed to administer the dose as soon as noticed, and resume the normal dosing schedule the next day1
For patients weighing less than 40 kg, the exact dose must be calculated
Myalept Dosage Adjustments and Discontinuation
In patients at risk for hypoglycemia
- Dosage adjustments, including possible large reductions, of insulin or insulin secretagogue may be necessary in some patients to minimize the risk of hypoglycemia1
- When treating patients concomitantly with Myalept and insulin therapy, close monitoring of blood glucose levels is recommended1
In patients at risk for pancreatitis
- Tapering of the dose over a 1-week period is recommended1
- During tapering, monitor triglyceride levels and consider initiating or adjusting the dose of lipid-lowering medications as needed1
Myalept Storage and Preparation
HCPs should support patient and caregiver training
Myalept storage and reconstitution
- For use in neonates and infants, reconstitute with preservative-free sterile WFI. When reconstituted with sterile WFI, Myalept should be administered immediately
- Unused reconstituted solution cannot be saved for later use and should be discarded
- For use in pediatric and adult patients, reconstitute with BWFI. When reconstituted with BWFI, Myalept solution can be used within 3 days when stored in the refrigerator between 36° F and 46° F (2° C and 8° C) and protected from light
- Discard unused reconstituted solution after 3 days. Attach the supplied sticker to the vial and enter the discard date
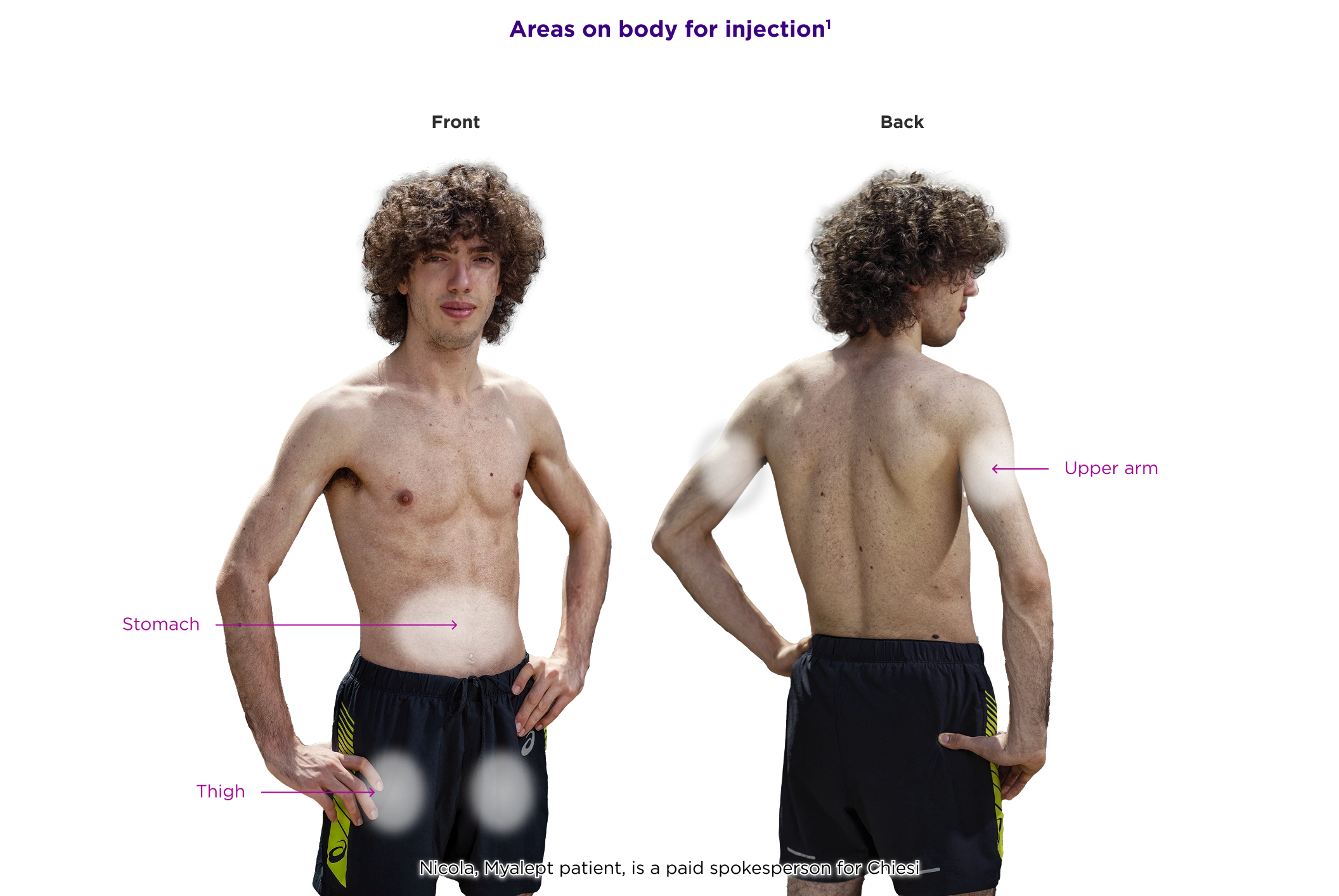
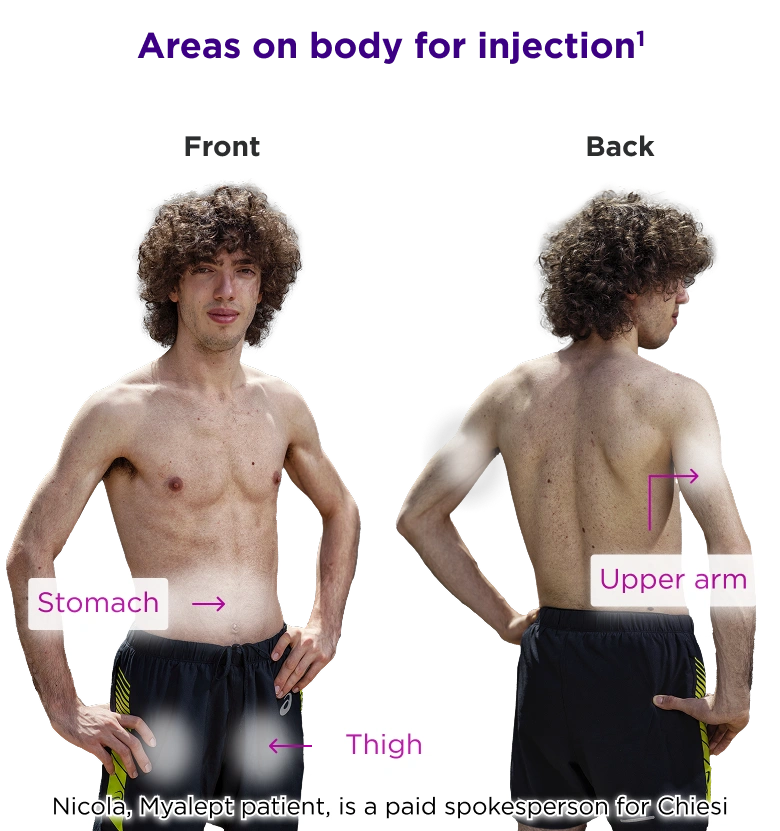
Myalept Administration
Myalept is given as a subcutaneous injection
Comprehensive Support Is Available for Your Patients
Get Support InformationINDICATION: Myalept® (metreleptin) for injection is a leptin analog indicated as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy.
LIMITATIONS OF USE: The safety and effectiveness of Myalept for the treatment of complications of partial lipodystrophy or for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established.
Myalept is not indicated for use in patients with HIV-related lipodystrophy. Myalept is not indicated for use in patients with metabolic disease, including diabetes mellitus and hypertriglyceridemia, without concurrent evidence of generalized lipodystrophy.
WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA
Anti-metreleptin antibodies with neutralizing activity have been identified in patients treated with Myalept. The consequences of these neutralizing antibodies are not well characterized but could include inhibition of endogenous leptin action and/or loss of Myalept efficacy. Severe infection and/or worsening metabolic control have been reported. Test for anti-metreleptin antibodies with neutralizing activity in patients who develop severe infections or show signs suspicious for loss of Myalept efficacy during treatment. Contact Chiesi Farmaceutici S.p.A. at 1-866-216-1526 for neutralizing antibody testing of clinical samples.
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy, both treated and not treated with Myalept. Carefully consider the benefits and risks of treatment with Myalept in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy.
Because of these risks associated with the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or Myalept and the risk for lymphoma, Myalept is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Myalept REMS PROGRAM.CONTRAINDICATIONS: Myalept is contraindicated in general obesity not associated with congenital leptin deficiency. Myalept has not been shown to be effective in treating general obesity. The development of anti-metreleptin neutralizing antibodies have been reported in obese patients treated with Myalept. Myalept is contraindicated in patients with prior severe hypersensitivity reactions to metreleptin or to any of its components.
WARNINGS AND PRECAUTIONS: A dose adjustment, including possible large reductions, of insulin or insulin secretagogue may be necessary in some patients to minimize risk of hypoglycemia. Closely monitor blood glucose in patients on concomitant insulin, especially those on high doses, or insulin secretagogue.
Cases of progression of autoimmune hepatitis and membranoproliferative glomerulonephritis (associated with massive proteinuria and renal failure) were observed in some patients with acquired generalized lipodystrophy treated with Myalept. A causal relationship between Myalept and the development and/or progression of autoimmune disease has not been established. Carefully consider the benefits and risks of Myalept treatment in patients with autoimmune disease.
Hypersensitivity reactions (eg, anaphylaxis, urticaria or generalized rash) have been reported. Patient should promptly seek medical advice about discontinuation of Myalept if a hypersensitivity reaction occurs.
Myalept contains benzyl alcohol when reconstituted with Bacteriostatic Water for Injection. The preservative benzyl alcohol has been associated with serious adverse events and death in pediatric patients, particularly in neonates and premature infants. Preservative-free Water for Injection is recommended for use in neonates and infants.
ADVERSE REACTIONS: Most common adverse reactions (≥10%) in clinical trials were headache, hypoglycemia, decreased weight, and abdominal pain.
Please see Full Prescribing Information, including Boxed Warning.INDICATION: Myalept® (metreleptin) for injection is a leptin analog indicated as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy.
LIMITATIONS OF USE: The safety and effectiveness of Myalept for the treatment of complications of partial lipodystrophy or for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established.
Myalept is not indicated for use in patients with HIV-related lipodystrophy. Myalept is not indicated for use in patients with metabolic disease, including diabetes mellitus and hypertriglyceridemia, without concurrent evidence of generalized lipodystrophy.
WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA
Anti-metreleptin antibodies with neutralizing activity have been identified in patients treated with Myalept. The consequences of these neutralizing antibodies are not well characterized but could include inhibition of endogenous leptin action and/or loss of Myalept efficacy. Severe infection and/or worsening metabolic control have been reported. Test for anti-metreleptin antibodies with neutralizing activity in patients who develop severe infections or show signs suspicious for loss of Myalept efficacy during treatment. Contact Chiesi Farmaceutici S.p.A. at 1-866-216-1526 for neutralizing antibody testing of clinical samples.
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy, both treated and not treated with Myalept. Carefully consider the benefits and risks of treatment with Myalept in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy.
Because of these risks associated with the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or Myalept and the risk for lymphoma, Myalept is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Myalept REMS PROGRAM.CONTRAINDICATIONS: Myalept is contraindicated in general obesity not associated with congenital leptin deficiency. Myalept has not been shown to be effective in treating general obesity. The development of anti-metreleptin neutralizing antibodies have been reported in obese patients treated with Myalept. Myalept is contraindicated in patients with prior severe hypersensitivity reactions to metreleptin or to any of its components.
WARNINGS AND PRECAUTIONS: A dose adjustment, including possible large reductions, of insulin or insulin secretagogue may be necessary in some patients to minimize risk of hypoglycemia. Closely monitor blood glucose in patients on concomitant insulin, especially those on high doses, or insulin secretagogue.
Cases of progression of autoimmune hepatitis and membranoproliferative glomerulonephritis (associated with massive proteinuria and renal failure) were observed in some patients with acquired generalized lipodystrophy treated with Myalept. A causal relationship between Myalept and the development and/or progression of autoimmune disease has not been established. Carefully consider the benefits and risks of Myalept treatment in patients with autoimmune disease.
Hypersensitivity reactions (eg, anaphylaxis, urticaria or generalized rash) have been reported. Patient should promptly seek medical advice about discontinuation of Myalept if a hypersensitivity reaction occurs.
Myalept contains benzyl alcohol when reconstituted with Bacteriostatic Water for Injection. The preservative benzyl alcohol has been associated with serious adverse events and death in pediatric patients, particularly in neonates and premature infants. Preservative-free Water for Injection is recommended for use in neonates and infants.
ADVERSE REACTIONS: Most common adverse reactions (≥10%) in clinical trials were headache, hypoglycemia, decreased weight, and abdominal pain.
Please see Full Prescribing Information, including Boxed Warning.